
Over the past weeks, BCI news has taken on a more patient-focused tone, with new implants and updates landing in quick succession. Neuralink is again the most visible example. In late January, Reuters reported that the company now has 21 participants enrolled in trials worldwide, up from the 12 people in September 2025. In the UK, seven GB-PRIME patients are now participating, all implanted between October and December 2025.
The global installed base of BCIs is still small, but it carries weight. Counting how many people live with a chronic, implanted BCI is a way of gauging where the field sits between proof and operations. Implant cadence, long-term follow-up, device stability, and everyday variability only become visible once multiple patients are enrolled in trials at the same time. But perhaps more importantly, as implants are used for extended periods, it becomes clear what real-life value the technology adds to patients' lives.
A chronic implanted BCI, as used here, refers to a brain interface intended to remain in the body and support ongoing use over months to years. The key attribute is consistent use: the implant is meant to be lived with, not briefly tested and removed. That definition is modality-agnostic. Intracortical arrays, subdural/epidural ECoG systems, endovascular implants, and other implanted sensors can all qualify, as long as they are used chronically.
Several high-visibility human milestones fall outside that boundary because they are temporary placements. Precision’s Layer 7-T sits in the “temporary but clinically integrated” category, with FDA documentation allowing use for less than 30 days. Paradromics’ first-in-human Connexus procedure was explicitly an implant-record-remove operation during epilepsy surgery, with removal in under 20 minutes. INBRAIN’s graphene-based implants are framed around surgical use cases with short-duration recordings, closer to intraoperative validation than chronic assistive deployment. These interfaces provide great value to the field, but they inflate an “implanted BCI” count in a way that does not match day-to-day life with an implant.
This boundary means the count is intentionally narrow. Several investigator-led programs and registry-listed trials involve chronically implanted neural interfaces, but do not publish auditable, up-to-date implantation totals. Others operate under academic protocols with different disclosure norms and reporting timelines. To avoid mixing estimated figures with confirmed numbers, the commercial tally below includes only known programs that have publicly stated how many people have been implanted orenrolled in a way that clearly implies implantation.
Neuralink’s well-known Telepathy program involves an intracortical, fully implanted BCI built for direct computer and robotic device control in people with severe paralysis. The system centers on the N1 implant, mounted in the skull and connected to ultra-thin electrode threads placed by Neuralink’s R1 surgical robot. The implant is designed to be wireless and rechargeable. Patients are typically shown using it as a computer input interface, translating movement intent into cursor control for everyday digital tasks such as typing, browsing, interacting with apps, and even playing games.
Neuralink operates across multiple countries, with implants reported in the United States, Canada, and the United Kingdom. The company implanted its first human participant in early 2024. It then expanded in the US before moving trials to Canada and the UK. UCL Hospitals says seven GB-PRIME patients have received the device, while counts of Canadian implants are unclear. For counting purposes, Reuters provides public anchors: 21 participants enrolled worldwide as of January 28, 2026, 12 people who received implants as of September 2025, and seven UK patients implanted since. A conservative lower bound is “at least 19,” with an upper bound of “up to 21,” reflecting the fact that enrolment does not always mean implantation.
Check out Neuralink's Patient overview.
Synchron’s Stentrode takes a different route: an endovascular implant delivered through the vasculature and deployed in a vessel near the motor cortex, avoiding open-brain surgery. The company positions it's device around chronic assistive computer control for people with severe paralysis, with clinical programs spanning the US and Australia. Functionally, Synchron’s public demos and reporting emphasize practical digital outputs, showcased in a demo with Apple devices, and includes hands-free navigation and selection on mainstream devices. Integrations lean on existing accessibility layers, rather than bespoke BCI-only software.
Synchron is one of the few companies to state an explicit chronic implant count. In its Series D announcement, the company said the Stentrode BCI had been implanted in 10 people with paralysis across its clinical trials. That figure serves as a fixed anchor for the commercial tally. In practice, the system is designed around stable, repeatable digital control in constrained interfaces, prioritising reliability over high-bandwidth outputs such as continuous speech decoding or free-form motor control seen in others.
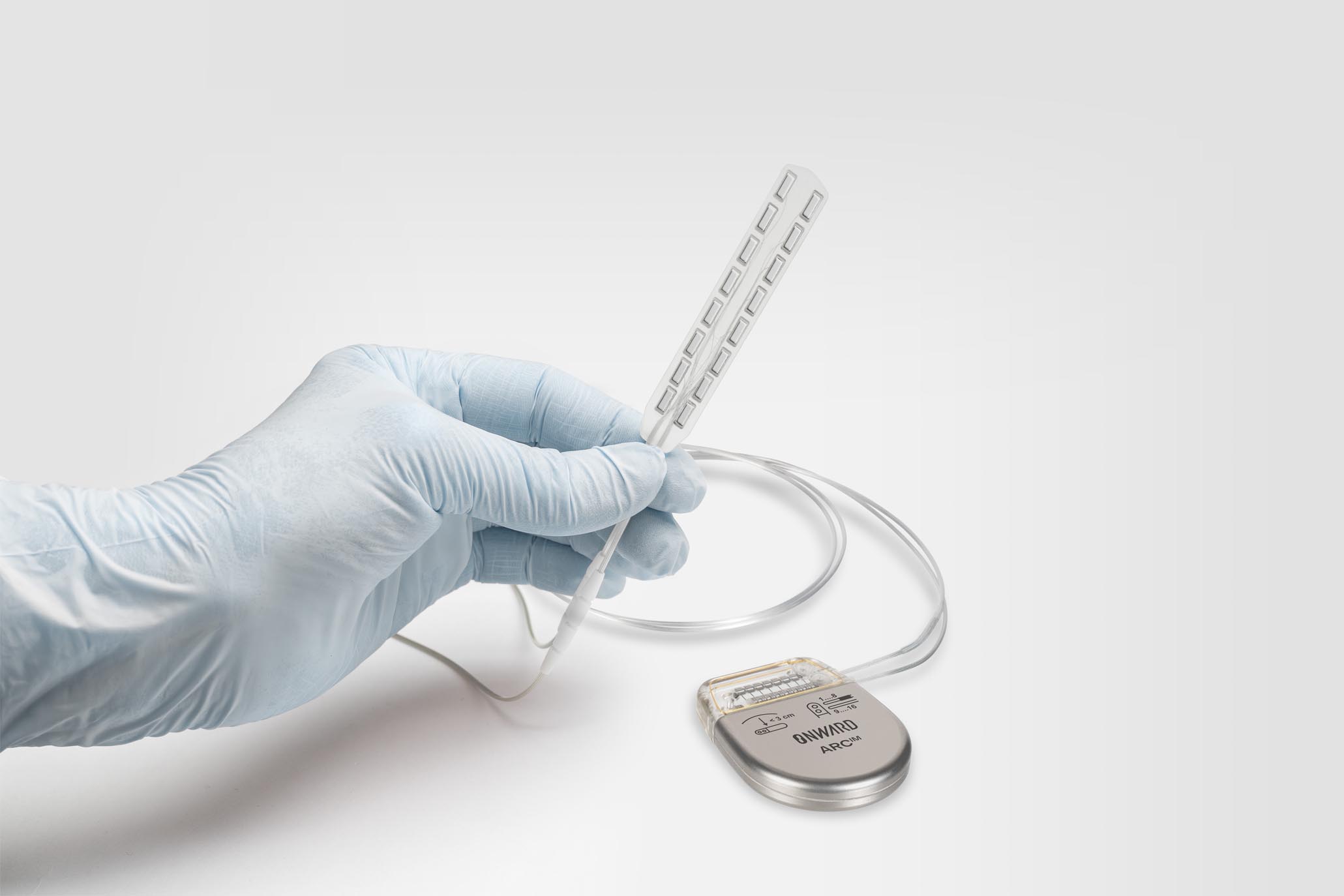
ONWARD Medical’s ARC-BCI sits closer to restorative neurotechnology than classic cursor control. The ARC-BCI system pairs an implanted brain interface with an implanted spinal cord stimulation system, aiming to translate decoded movement intention into stimulation patterns that restore thought-driven movement after paralysis. The implant is placed epidurally on the motor cortex to record signals associated with movement intention, with AI decoding and wireless transmission to an implanted neurostimulator.
In its January 2026 update, ONWARD reported two additional ARC-BCI implants performed at CHUV in Lausanne, bringing the total number of implanted patients to seven. The procedures targeted different restoration goals, spanning upper- and lower-limb movement, and build on more than eight years of human safety data for the BCI component.
CorTec develops the Brain Interchange system, designed as a fully implantable, wireless interface that supports both chronic recording and stimulation. The platform is framed around therapeutic, closed-loop applications rather than assistive typing or cursor control alone. In its current clinical focus, Brain Interchange is being evaluated in stroke rehabilitation, where cortical signals are recorded and used to drive stimulation patterns intended to support neuroplasticity and functional recovery over time.
With this week's announcement, CorTec’s human program now moves beyond a single first-in-human milestone. The company first reported a successful implantation in July 2025 under an FDA IDE, and has now confirmed a second patient has received the implant. CEO Frank Desiere tells Neurofounders that a third implant planned is planned in March. That moves Brain Interchange into a small group of BCIs that are beginning to accumulate multiple chronically implanted users, and shifts the emphasis from technical feasibility toward repeatability, follow-up, and longitudinal use in a therapeutic setting.
In China, the implanted-BCI landscape has two companies that list publicly quoted patient counts. WIRED reports that NeuCyber NeuroTech’s coin-sized Beinao-1 has been implanted in five people with paralysis, with recipients using it to move a computer cursor and navigate smartphone apps. Reuters previously reported three implanted patients as of March 2025 alongside an explicit plan to scale toward larger trials, which helps anchor the trajectory even when updates are episodic and hard to verify.
WIRED also reports that NeuroXess has implanted six paralyzed patients, including for the use of speech decoding in three and thought-based control of digital devices in the others. That adds 5 (NeuCyber) and 6 (NeuroXess) to the commercial bucket. However, public trial documentation and consistent registry reporting is less standardized than in US and European programs. Hence, while the counts in the listed reporting statements can be included, extrapolating beyond them based on company statements is not done here.
Many of the longest-running implanted BCIs sit outside commercial programs. Academic studies have accumulated years of experience with chronic implants, often using percutaneous connectors and bespoke hardware that would be difficult to productize as-is. These systems can support long follow-up and rich experimentation, but they operate under different constraints. Disclosure norms are uneven, patient counts are spread across publications rather than press releases, and success is measured in scientific insight rather than repeatable deployment. For that reason, academic implants are best treated as a separate bucket rather than folded into a commercial tally.
That bucket includes several well-established lines of work. Chronic intracortical programs such as BrainGate have supported communication and cursor control in people with tetraplegia for years, with participants often living with implants for multiple years. Surface-based approaches also appear, including chronic ECoG systems used for communication in people with locked-in syndrome. A concrete example is the Utrecht NeuroProsthesis work, where implanted recordings have been used to enable letter selection and communication in patients with severe paralysis under long-term research protocols.
Putting a number on this academic population is hard. A 2024 Nature Reviews Bioengineering review provides one of the clearest syntheses of the academic implanted BCI landscape, summarizing active and completed trials across modalities and reporting an order-of-magnitude count of 67 active participants between 1998 and 2023. That figure is best treated as a baseline rather than a live total. Some programs have added participants since, others have concluded, and reporting lags make a precise 2026 update unrealistic. The point of including it here is context, not precision.
Taken together, the estimates of commercial and academic implants sketch the current scale of BCI's implanted base. On the commercial side, the conservative lower bound counts only people explicitly reported as having received implants: Neuralink (at least 17), Synchron (10), ONWARD (7), CorTec (2), NeuCyber (5), and NeuroXess (6), for a total of 47. The upper bound treats “enrolled” figures as implanted where strongly implied, pushing the commercial total to up to 51 or higher, driven primarily by Neuralink’s 21 reported participants. On the academic side, the Nature Reviews Bioengineering review points to 67 implanted participants up to late 2023, with the clear caveat that this is a dated baseline and a longitudnal measure.
And so, where not a single patient lived with a commercial-implanted BCI just a few years ago, the global population of people living with a chronic implanted BCI is now at least fifty, nearing one hundred. The transition from research to operations is now visible not just in demos and press releases, but in the counts of real people using BCIs to materially improve their lives.
[Cover image: Neuralink]