When Synchron announced a $200 million Series D in November 2025, the neurotechnology community’s attention centered on the large valuation and increasing capital velocity. But what mattered more than dollar amounts is what that funding backs. Synchron is developing an endovascular brain-computer interface, implanted via the brain’s blood vessels rather than through open-brain surgery. Instead of following the dominant trajectory in BCI development, the company chose not to prioritize maximum signal fidelity via direct cortical penetration.
Instead, it deliberately selected a lower-fidelity approach. This choice reflects not a technical constraint, but a prediction about where brain-computer interfaces will encounter friction as they move toward clinical deployment. As systems leave controlled research settings, considerations such as procedural risk, clinical workflow, and available expertise begin to shape what is feasible at scale. That framing reshapes how Synchron approaches hardware design, decoding strategy, and clinical integration, and it provides the lens through which the system itself is best understood.
Discussions of brain-computer interface technology typically emphasize decoding sophistication, including neuron yield, information bandwidth, and the complexity of motor parameters captured. Much of the field has implicitly treated signal fidelity as the primary optimization target. Over time, this focus has shaped both research priorities and expectations around what constitutes technical progress.
Conventional intracortical BCIs, however, rely on craniotomy. A neurosurgeon creates a surgical opening, exposes the motor cortex, and places electrodes directly into neural tissue. The procedure carries meaningful clinical risk, including hemorrhage, infection, and cortical damage, and it depends on highly specialized surgical expertise. In most developed healthcare systems, craniotomies are performed at limited volume and are reserved for conditions such as tumor resection or epilepsy management. That reality places practical limits on how widely elective brain implants can be deployed for motor restoration.
From that perspective, scalability is constrained less by decoding performance than by procedural capacity. The challenge is not one that can be resolved through incremental technical refinement alone; it is embedded in how care is delivered and who is trained to deliver it.
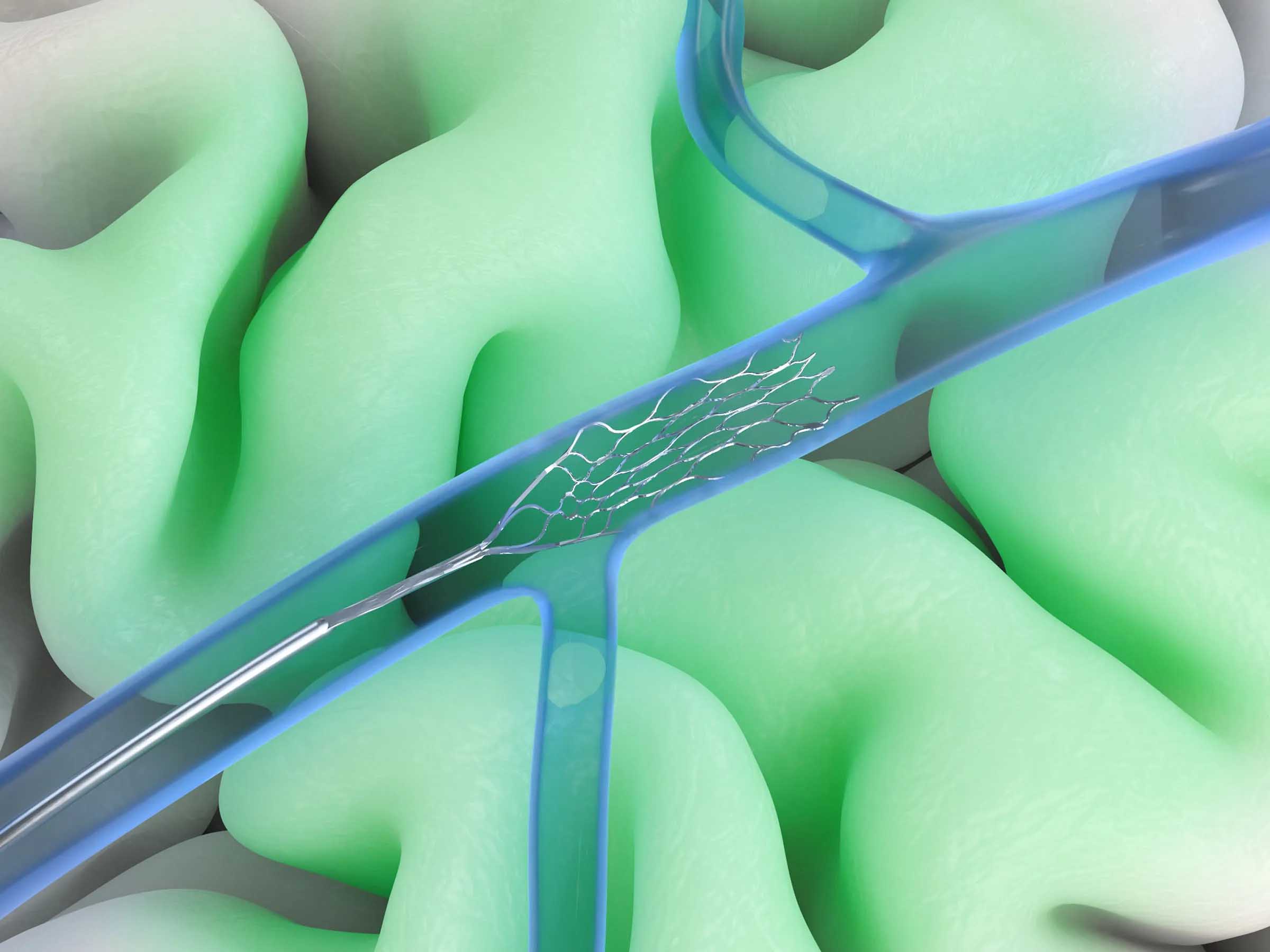
Synchron’s endovascular strategy is built around a different clinical pathway. An interventional radiologist, a clinician already performing high volumes of catheter-based procedures, advances the Stentrode through the jugular vein into the superior sagittal sinus, the major venous drainage from the motor cortex. The procedure takes approximately 20 to 30 minutes under conscious sedation in a standard radiology suite, with patients typically discharged the following day.

The required infrastructure is already widespread, and the relevant clinical workforce is already in place. Within that context, the approach reflects a deliberate engineering orientation rather than a trade-off made under constraint. As CEO Thomas Oxley has put it, “We’re not trying to build the best BCI. We’re trying to build a BCI that actually gets implanted.” The statement captures how considerations of deployment, rather than theoretical performance alone, shape the system’s design.
Synchron's Stentrode embodies this philosophy at the hardware level. The device measures approximately 40 millimeters in length and is constructed from nitinol, a nickel-titanium alloy long used in vascular implants. Its flexible mesh design allows it to conform to the vessel wall without obstructing blood flow, while 16 platinum electrodes are distributed along its surface at three-millimeter intervals.
That electrode density reflects how the system is intended to be used. Intracortical electrode arrays can support hundreds or thousands of channels, each capable of isolating activity from individual neurons. By contrast, the Stentrode’s 16 channels capture only population-level signals, aggregating activity across groups of neurons rather than resolving single-unit structure. As a result, fine motor parameters such as individual finger kinematics or precise grasp-force dynamics fall outside the system’s resolution.
For the applications Synchron is targeting, that level of detail is not required. Tasks such as text entry, cursor control, digital device navigation, and smart home operation rely on discrete selections or sustained motor intentions rather than continuous, high-dimensional motor control. Within that use-case envelope, population-level signals are sufficient.
With hardware design bounded by procedural and anatomical constraints, engineering effort shifts toward decoding methodology. Raw voltage signals from the Stentrode’s 16 channels are filtered to remove physiological artifacts and noise, then processed through feature extraction and classification pipelines. Synchron has integrated NVIDIA’s Holoscan platform to run inference on-device rather than relying on cloud connectivity, reducing latency and limiting dependence on external infrastructure for real-time command execution.
Recent advances in machine learning provide additional leverage at this stage. Rather than initializing decoding models from scratch for each patient, parameters can be informed by data learned across prior implants and then adapted to an individual’s neural signature. This approach reduces per-patient calibration time and allows users to reach functional operation more quickly. In a system designed for scale, those efficiency gains become increasingly consequential.
Clinical evidence for this decoding strategy comes from the COMMAND trial. Six participants with severe bilateral upper-limb paralysis due to ALS or spinal cord injury received Stentrode implants. The primary endpoint was safety, with no device-related serious adverse events reported through 12 months. Across the cohort, there were no thrombotic events, hemorrhage, infections, or device migration, and follow-up imaging confirmed stable positioning and preserved vessel patency.
Functional outcomes indicated reliable motor-intention decoding. Participants achieved cursor-control accuracy above 90 percent, with text entry rates of 14 to 20 characters per minute without predictive assistance. Users were able to navigate email, online banking, messaging platforms, and smart home interfaces. Most reached independent home use within two to three months of implantation, suggesting that the decoding paradigm is intuitive and converges with continued use.
Beyond performance metrics, these results translated into meaningful changes in daily life. One participant returned to part-time employment, while another reestablished regular communication with family via email after years of complete motor paralysis. Taken together, the outcomes point to clinical utility grounded in reliability and usability rather than maximal decoding precision.

Against that technical and clinical backdrop, Synchron secured a $200 million Series D last November, bringing total funding to $345 million and implying a valuation of approximately $1 billion. The investor base, which includes the Australian National Reconstruction Fund, Qatar Investment Authority, Protocol Labs, Khosla Ventures, Bezos Expeditions, and ARCH Ventures, reflects confidence in the company’s deployment-oriented strategy rather than in incremental gains in neural resolution alone.
Leadership has revealed three priorities, now that funding is in place: advancing pivotal trials toward FDA approval, preparing commercial launch infrastructure, and expanding artificial intelligence and engineering capacity. The company has established a new engineering hub in San Diego focused on next-generation device development, while a cognitive AI team in New York is working on decoding more complex neural states beyond motor control, including language-related intention and memory-associated signals.
Regulatory clearance will depend on a pivotal multisite trial designed to support either 510(k) clearance or de novo classification. To date, no chronically implanted brain-computer interface intended for long-term assistive use has received FDA market approval. If Synchron's pivotal trial is successful and review timelines follow historical norms, initial commercial deployment would begin at academic medical centers and specialized neurotechnology clinics, with subsequent expansion to regional interventional radiology centers equipped with trained operators.
The next 12 to 24 months will test whether Synchron’s constraint-driven approach can translate into clinical adoption. Regulatory approval represents one milestone, but broader uptake will depend on additional factors, like the ability to train interventional radiologists at scale, establish reimbursement pathways, and integrate BCI workflows into routine hospital operations. Patient outcomes will also need to justify implantation as non-invasive alternatives continue to improve.
At a system level, Synchron’s strategy emphasizes alignment with existing clinical infrastructure rather than maximal technical performance. By prioritizing deployability and procedural compatibility, the company has designed its platform around conditions that could support wider access. In medical device markets, such alignment often shapes whether technologies move beyond early clinical use into broader practice.
The recent Series D provides the capital required to pursue this pathway through pivotal trials and early commercialization. Whether the approach proves durable will depend on execution across regulatory review, clinical integration, and real-world outcomes. The strategic framing is well defined, but its effectiveness will ultimately be determined in practice.
[Image credit: Synchron]