The blood–brain barrier (BBB) is one of biology’s most effective protective systems; composed of tightly joined endothelial cells and supported by astrocytes and pericytes, it carefully regulates what passes from the bloodstream to the brain. This same selectivity, however, prevents many drugs from reaching their targets at effective concentrations, causing promising candidates to fail despite validated biology and contributing to up to 95% attrition observed in CNS drug development.
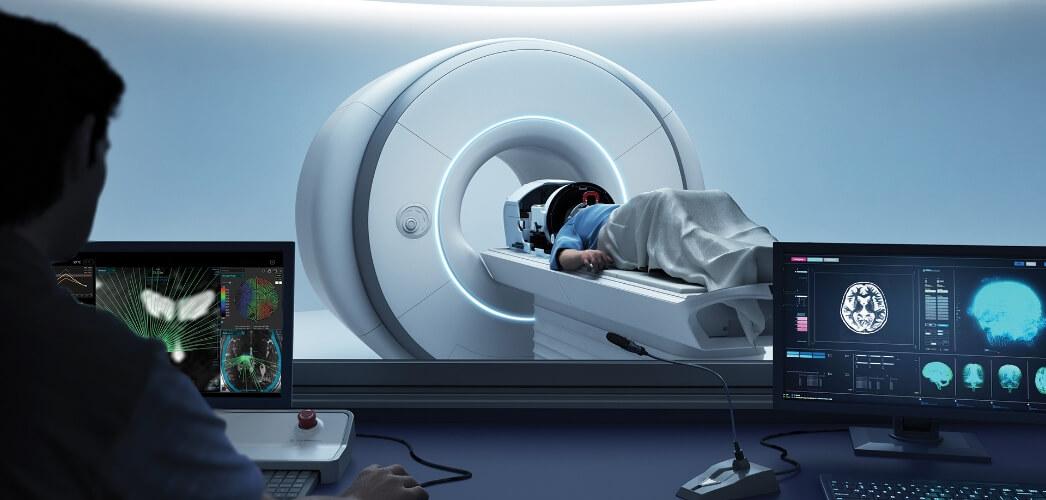
Over the past decade, focused ultrasound (FUS) combined with intravenously administered microbubbles has emerged as the most credible strategy to modulate BBB permeability transiently and locally. Low‑intensity, pulsed ultrasound induces controlled microbubble oscillations that exert mechanical forces on the vascular endothelium, creating a window for drug entry without substantial tissue damage. As the field moves to less invasive techniques, the challenge has shifted from demonstrating access to achieving robust, repeatable, and scalable BBB modulation across a diverse population of patients.
In current clinical research, glioblastoma and Alzheimer’s disease have increasingly served as testbeds for BBB modulation. In both areas, therapeutics with validated targets exist, but limited brain exposure continues to constrain clinical impact.
In Alzheimer’s disease, monoclonal antibodies targeting amyloid-β or tau typically reach the brain only in very small amounts, around ~0.1–0.3% of plasma exposure, limiting their ability to engage neuronal and synaptic targets. In glioblastoma, the situation is different but related: although the BBB is often disrupted in the tumor core, the infiltrative cells responsible for recurrence remain shielded behind an intact barrier, preventing therapeutic concentrations from reaching the most clinically relevant regions.
Beyond drug delivery, transient BBB opening has also been linked to short-term neuroimmune and vascular responses. Early clinical studies have reported microglial activation, changes in amyloid burden, and shifts in inflammatory pathways in Alzheimer’s disease, while in glioblastoma BBB disruption may enable immune cell infiltration and modify aspects of the tumor microenvironment. Whether these acute biological effects translate into meaningful therapeutic benefit remains an open question.
Clinical efforts to modulate the BBB using focused ultrasound have progressed through a series of trade-offs. Early approaches favored implantable systems that enable more repeatable acoustic delivery by bypassing skull-induced attenuation and variability. More recent efforts have shifted toward non-invasive approaches that prioritise procedural simplicity and scalability.
Carthera represents one of the most advanced regulatory efforts in BBB modulation, using an implantable focused ultrasound device designed for glioblastoma. After a Phase I/II study demonstrating safety and improved chemotherapy delivery, Carthera advanced into SONOBIRD, a large randomized Phase II/III trial enrolling roughly 560 patients, with enrollment expected to complete by the end of 2026. Although the device requires neurosurgical implantation, median survival in recurrent glioblastoma remains under one year, a level of unmet need that continues to justify more complex and invasive strategies.
Insightec marks the transition to fully non-invasive, transcranial focused ultrasound (tFUS). Its MRI-guided platform allows real-time monitoring of targeting, safety, and BBB permeability. The system is FDA-approved for essential tremor and is currently being evaluated in a Phase III trial in brain metastases in combination with immunotherapy, with completion targeted for the end of 2027. By removing the need for implantation, Insightec reduces procedural burden, but still depends on MRI infrastructure for guidance and treatment delivery.
NaviFUS represents a further simplification of the non-invasive tFUS workflow. The platform uses neuronavigation-guided targeting based on pre-acquired MRI or CT scans, avoiding real-time MRI during treatment while remaining transcranial and non-implantable. This reduces procedural complexity compared with MRI-guided systems, but does not fully resolve the challenge of individualized acoustic control. NaviFUS has entered early feasibility and Phase I studies in small glioblastoma cohorts and remains at an early stage of development.
As the field moves toward non-invasive tFUS, inter-individual anatomical variability has become the dominant barrier. Differences in skull thickness, density, and heterogeneity distort ultrasound propagation, reduce delivered energy, and narrow the margin between stable microbubble oscillation and harmful collapse. Importantly, this threshold varies across patients and across brain regions.
Maintaining microbubbles within a stable oscillation regime is therefore critical. Exceeding a narrow acoustic window can trigger bubble collapse and cause injury. While platforms such as those developed by Insightec and NaviFUS increasingly combine pre-procedure imaging with real-time feedback to personalise exposure, this added complexity and procedural burden limit scalability.
Recent work published in January 2026 by teams from BioMaps (SHFJ), BAOBAB (NeuroSpin), and CEA-Jacob introduced a skull-aware adaptive control algorithm that adjusts ultrasound delivery based on each individual’s frequency-dependent skull attenuation. The approach filters out ultrasound signals generated outside the brain, which can otherwise obscure activity at the intended target. By isolating the relevant signal, the method enables more reliable bubble control while reducing reliance on extensive MRI- or CT-based personalisation.
Reducing dependence on imaging represents a key step toward fully non-implantable transcranial systems. While technically demanding, such approaches are far more scalable and are likely to be essential for repeatable, low-burden interventions across neurological and psychiatric indications.
[Cover image credit: Insightec]