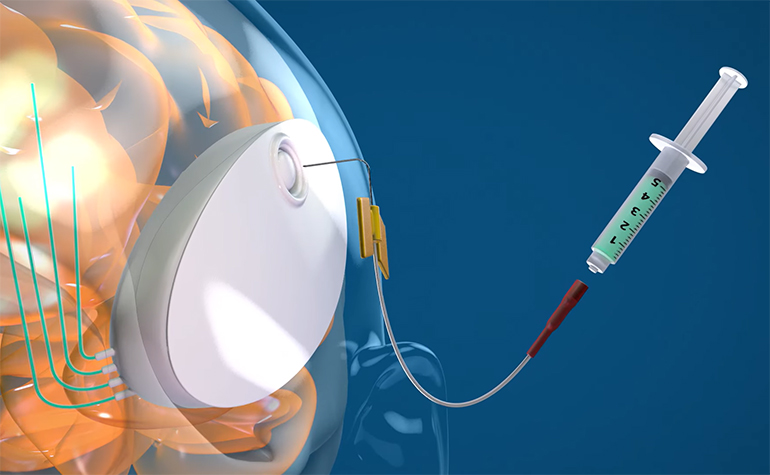
CraniUS Therapeutics has closed a $20 million Series B to scale development of its implantable NeuroPASS platform, scaling up regulatory preparation, manufacturing, and product testing. The company is building a skull-embedded drug delivery system designed to provide direct, repeatable access to brain tissue. NeuroPASS embeds a cranial implant with intracranial catheters, creating a fixed access point for targeted drug delivery and monitoring.
For many CNS therapies, access through the blood-brain barrier (BBB) is the limiting constraint. Even when compounds are promising, delivering them to precise brain regions repeatedly is operationally difficult. NeuroPASS, a chronic, implanted interface that stays in place once inserted, is designed around that problem. The premise is that durable access, once established, can support ongoing drug administration and adjustment within standard clinical workflows.
CraniUS’ Series B totals $20 million, consisting of $19 million from private investors and $1 million in non-dilutive funding from the State of Maryland. CraniUS says the raise brings total capital to approximately $40 million and is intended to fund operations into 2027, including financing regulatory submissions, manufacturing scale-up, and product testing. NeuroPASS remains investigational and has not been FDA approved or cleared for clinical use.
The company frames the round as validation of its access-first thesis. “This is not an incremental advance, it represents a fundamentally new approach to treating neurological disease and brain cancer,” said co-founder Dr. Chad Gordon. “We set out to build a platform that could help address a therapeutic barrier that has limited care for decades.”
The round follows CraniUS’s $19.4 million Series A in September 2022. That financing positioned the company around chronic, direct drug delivery to the brain using a pump-based system with wireless charging and convection-enhanced delivery. The goal is to expand that platform with multiple product and use extensions. CraniUS was founded in 2021 by Gordon, CMO, and CTO Deborah Weidman, with Michael Maglin appointed CEO as the program advanced toward a first-in-human clinical study.
As of the current update, CraniUS reports 15 issued U.S. patents alongside additional international filings supporting its platform and product roadmap. The company traces its technical origin to Gordon’s work on the temporal cranial space and the recurring clinical failure of therapeutics that do not reach brain tissue due to the blood-brain barrier. The near-term challenge is execution; translating their access thesis into consistent delivery performance, a scalable manufacturing process, and a viable outpatient workflow as the platform moves through regulatory review.

NeuroPASS is built around a skull-embedded implant, embedded under the scalp and connected to intracranial catheters that deliver drugs directly into brain tissue. Delivery is pump-driven and controlled, with the system designed to be refillable and supported by wireless charging so dosing can be repeated without reinstalling hardware. The company frames this around the concept of convection-enhanced delivery (CED), where a pressure gradient drives flow through distributed spaces to spread drugs beyond what diffusion-limited injection can typically achieve.
That design choice is required for the chronic use CraniUS is after. Externalized catheters increase infection risk and are difficult to sustain for months-long care, while less sophisticated implanted approaches can make it hard to adjust dosing once a regimen changes. A fixed cranial access point solves those issues while shifting focus to ongoing management, where flow rate and dosing schedules can be treated as long-term adjustable parameters.
CED is not a new concept. Since its introduction in the 1990s, work has shown why it remains attractive but also why it has been hard to translate into routine care. Distribution is highly sensitive to catheter placement, tissue properties, and infusion parameters, and backflow (reflux) along the catheter insertion tract can undermine targeting. Many CED programs therefore pair delivery with reflux-mitigation hardware and in vivo verification of distribution, often using co-infused surrogate tracers and imaging proxies. NeuroPASS is an attempt to productize that delivery mode into an implantable platform.
There is also another method for opening the BBB gaining a lot of attention: transcranial focused ultrasound (tFUS), typically paired with injected microbubbles, to transiently increase permeability in a targeted region without placing a catheter into brain tissue. tFUS is a session-based approach, often imaging-guided, where targeting, parameters, and timing relative to systemic dosing determine success. That makes it attractive for repeat treatments without implanted intracranial hardware, but it keeps delivery coupled to systemic pharmacokinetics and the limits of a temporary opening window. NeuroPASS is an alternative route: it bypasses BBB opening entirely by implementing fixed access infrastructure to enable repeated delivery.
[Image credit: CraniUS Therapeutics]