
Neuralink has completed its first implant in the United Kingdom at University College London Hospitals. The participant, identified as Paul and living with motor neurone disease, was able to control a computer within hours after surgery. This is the company’s first public human implant outside North America, and it places the work inside an NHS setting.
As of early September, Neuralink said 12 people had received its implant globally. Canada then recorded its first two surgeries at Toronto Western Hospital in late August and early September, and the United Kingdom followed with the UCLH procedure in October. Taken together, the total now stands at at least 14 recipients, reflecting multi-country execution and a program moving from single-site feasibility toward a broader clinical footprint.
London is now part of the story. In October at UCLH’s National Hospital for Neurology and Neurosurgery, surgeons implanted Neuralink’s device in a patient, Paul, living with motor neurone disease. Within hours, he was moving a cursor by thought. Since receiving the implant, Paul has been working with Neuralink engineers to explore uses that enrich daily life and restore autonomy, with follow-up appointments and research sessions planned as he learns to use the device. UCLH posted the confirmation on 27 October.
The procedure sits inside GB-PRIME, the United Kingdom’s early feasibility study of the N1 implant. N1 is the fully implantable, intracortical unit that records through hair-thin polymer threads placed in the motor cortex and streams signals to external software for decoding. The UK protocol evaluates the safety and basic functionality of the implant in people with severe paralysis and is designed to test whether this pairing (of the implant and its accompanying surgical robot, R1) can deliver reliable, controllable signals in routine clinical sessions.
UCL and Newcastle are Neuralink's two partners for this phase. UCLH brings a large, research-active neuroscience hospital with neuro-surgery, rehabilitation and ethics infrastructure under one roof. Newcastle Hospitals adds a second leading NHS centre with established neurotechnology programs and study operations. Both institutions have published public materials describing the aims of GB-PRIME and their roles in it.
Enrollment in the GB-PRIME study is not closed. People over 22 with qualifying paralysis can still register interest through Neuralink’s patient registry.
The London result shows again that the N1 implant and R1 robot pairing can provide real clinical value. Reproducing same-day control in a new hospital suggests that the placement workflow is portable and that decoding can reach utility without long calibration sessions. In practice, that is what progress looks like for an intracortical BCI: threads where you intend them, stable signal quality session to session, and a swift path from operating theatre to routine clinic.
Getting here took a few hard turns. The N-1 program moved from FDA go-ahead to the first U.S. implant, encountered early thread retraction in patient one, and then adjusted both software and surgical approach before proceeding. By late spring 2024, the FDA cleared a second implant, and by August, the company said the second participant did not have the retraction issue. Neuralink’s own 100-day update defined the user-experience metrics that now anchor external expectations, from bitrate and click accuracy to the kinds of tasks that feel like independence rather than a demo clip.
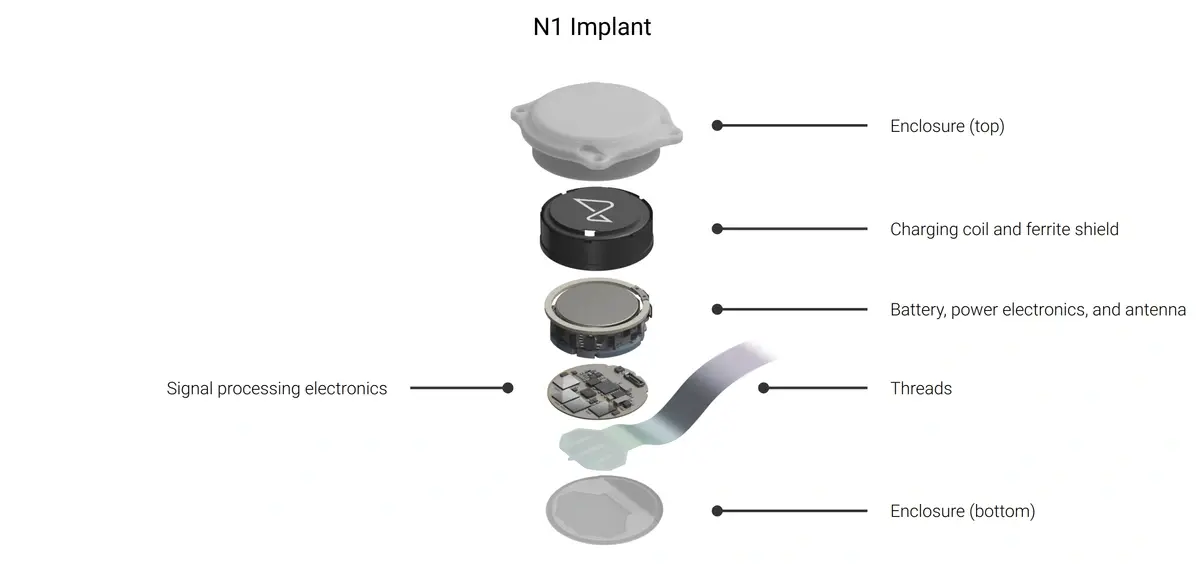
So where does N1 stand today. Early feasibility studies are built to answer two questions first: is the device safe to live with, and can it deliver controllable signals reliably? PRIME in the U.S. and GB-PRIME in the U.K. describe exactly that scope, with the N1 as the fully implantable, wireless intracortical unit and R1 as the robot that places hair-thin polymer threads in motor areas. The practical baseline to watch is calibration time to useful control, stability across weeks of sessions, and whether tasks map to day-to-day independence and utility rather than lab-based performance on pre-determined tasks.
What comes next is already sketched in the study and regulatory plans. In the United States, PRIME continues as an FDA IDE early feasibility study, with additional participants following the second implant and the aim of building evidence that can support a future pivotal stage on the road to approval. In the United Kingdom, GB-PRIME proceeds at UCLH and partner sites under MHRA and ethics approvals, with multi-site execution designed to show reproducibility in routine NHS care. In Canada, CAN-PRIME is active at Toronto Western Hospital, which completed the first two procedures outside the U.S. in late August and early September and is set up to keep enrolling.
Across these tracks, the likely near-term cadence is more participants, periodic study updates, and incremental publication of safety and functionality data that converge toward the larger regulatory filings ahead. The London implant is the clearest sign yet that N1 can be reproduced in new health systems, under NHS rules, with same-day utility. If that performance sustains as the UK cohort grows, it strengthens Neuralink’s bid to be first to a clinically approved intracortical BCI.