.jpg)
Europe’s neurotech scene has been brilliant in labs and quiet in clinics. That balance shifted this week in Munich, where a hospital-led team implanted an invasive brain-computer interface into a quadriplegic patient. The U.S. has dominated headlines through startup-led programs; Germany’s move signals a clinic-first posture, embedded in a university hospital, oriented to function, and built to produce the kind of evidence healthcare systems will ask for.
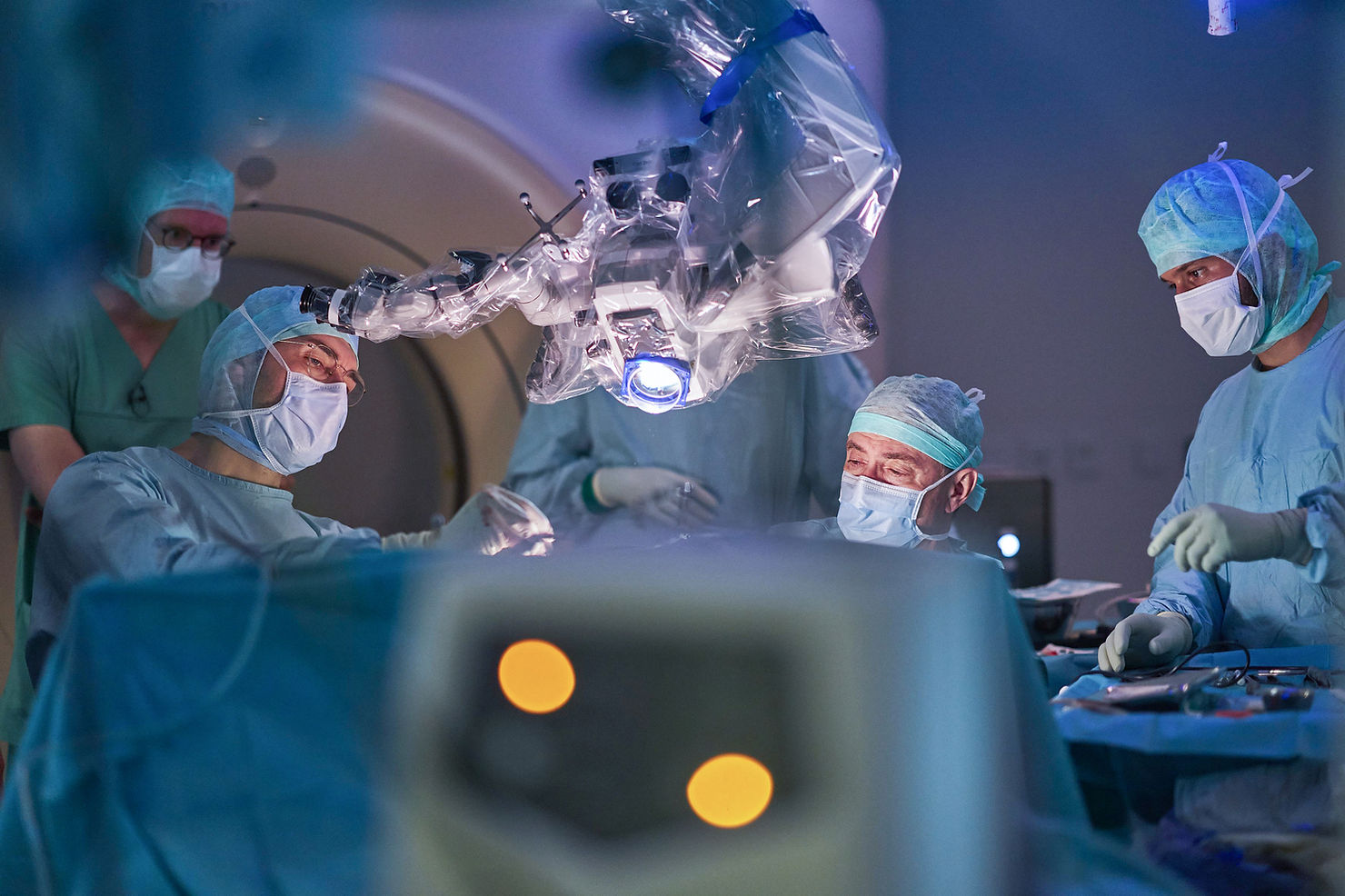
At the Technical University of Munich's university hospital, surgeons completed a five-hour operation to implant a 256-microelectrode intracortical interface in a 25-year-old with quadriplegia. The study now shifts to twice-weekly sessions to train AI decoders for cursor control and mouse clicks before stepping toward smartphone use and robotic grasp. The team frames the effort explicitly as closing Europe’s BCI gap to U.S. centers, while keeping the emphasis on research, safety, and rehabilitation-relevant outcomes.
Surgeons at TUM’s university hospital, Klinikum rechts der Isar, implanted the 256-channel intracortical brain-computer interface in a 25-year-old man with quadriplegia. The procedure lasted a little over five hours and targeted cortex involved in planning and executing grasping movements. TUM characterises the operation as the first of its kind in Europe in a patient with quadriplegia.
With surgery complete, the study has moved into decoder training. The participant meets the team twice a week, connecting via a percutaneous port so AI models can learn to associate neural activity with intended movements. Initial milestones are cursor control and mouse clicks, followed by smartphone use and stepwise control of a robotic arm. After the first sessions, the group reports an early signal in which the decoder can infer intended cursor direction while the participant tracks a moving target.
TUM places this within a broader clinical neurotechnology program. The team notes prior work in 2022 using a similar device in a stroke patient to map language processing in the intact right hemisphere. The current spinal cord injury study is led by the Department of Neurosurgery with robotics support from the Munich Institute of Robotics and Machine Intelligence. The study is framed as a bid to close the gap with leading U.S. centers by concentrating medicine, neuroscience, AI and engineering on one campus.

TUM’s “first” is worth defining precisely. It refers to an intracortical BCI in a person with quadriplegia. Europe already has implants on record in adjacent contexts, including Clinatec’s epidural ECoG controlling a four-limb exoskeleton in tetraplegia and Wyss Center with the University of Tübingen using intracortical arrays to enable communication in complete locked-in ALS.
The operating model matters as much as the headline. TUM's program sits inside a university hospital, advances through decoder training and rehab tasks, and is judged against endpoints that health systems care about, like activities of daily living and caregiver time. U.S. programs led by startups are often optimised for speed and frequent public updates. The comparison is context, not a ranking, but it explains why Europe’s announcements tend to arrive later and with tighter clinical scaffolding.
What makes this moment timely is how it fits the stack Europe is assembling. Clinatec’s WIMAGINE supplies a stable cortical readout. ONWARD’s implanted systems act at the level of the spine. EPFL and CHUV have shown a working bridge between the two in humans. Now, Munich adds a higher-fidelity control channel at the cortex that can sharpen intent. If those signals remain robust through training and everyday variability, the path from lab systems to prescribable tools becomes easier to see.
Europe’s play is hospital-first, and the consequences are practical. Studies are performed inside university clinics and have to fit real rehab schedules, staffing, and infection-control protocols. Success is judged on tasks that matter to care teams and eventual payers: computer access at home, time saved for caregivers, independence in daily routines, and whether the device can be supported by a hospital service department. That lens is different from a feasibility demo; it is about something a clinic can actually run week after week.
Regulation and payment push in the same direction. In the EU, MDR requires a CE mark backed by clinical evidence and post-market surveillance through notified bodies. After that, national health systems ask for health-technology-assessment-style data before routine use. The bar is not just “works once,” but “works safely and repeatably, can be maintained, and has a service plan.”
In the U.S., FDA IDE and Breakthrough programs encourage early feasibility, and visibility often runs through company updates and conference data while reimbursement pathways are negotiated later. The end goals overlap, but Europe front-loads evidence that fits into public healthcare.
Industry alignment is beginning to reflect these differences. European teams are stitching together a clinical stack: cortical intent capture in hospitals, spinal stimulation where appropriate, and assistive robotics configured for therapy services.
The commercial work is unglamorous but decisive: manufacturing that meets hospital procurement rules, sterilisation and field-service playbooks, training for therapists, and software that passes IT security reviews. Venture capital in the U.S. still outmuscles Europe, but Europe’s customers are hospitals and payers; products that plug into their workflows can move quickly once the evidence lands.
Two near-term paths follow. If month-to-month performance stays stable in multiple participants, session time remains manageable, and safety stays clean, Europe has a credible route from study kit to a prescribable system inside public hospitals. If signal quality drifts, training proves too heavy, or ports complicate care, attention will tilt back to less invasive interfaces. Either way, the gap that matters is no longer publicity. It is which model can turn neural control into a service a hospital can run and a payer can justify.