
Psychiatry has spent decades treating broad diagnoses that do not map cleanly to brain biology. The result is uneven outcomes among patients and trial programs that struggle to separate responders from non-responders. That model is starting to crack. Alto Neuroscience just paired a positive FDA meeting with a $50 million private placement to speed development of its depression program, a signal that precision psychiatry is becoming a clinically validated reality.
Precision psychiatry means matching patients to therapies using measurable brain and behavior signals, rather than symptoms alone. Alto’s platform collects EEG readings, computerized cognitive task performance, and wearable data to stratify patients and guide study design. With fresh capital and FDA alignment, Alto now becomes a live test of whether biomarker-guided selection can speed and sharpen psychiatric drug development.
Alto Neuroscience raised $50 million in a private placement led by Perceptive Advisors, stating that the proceeds will accelerate the development of its ALTO-207 compound for treatment-resistant depression. The financing was announced alongside a company update that it has aligned with the FDA on the next steps for the drug-development program.
Following the recent FDA meeting, Alto says it plans to start a Phase 2b study for ALTO-207 by mid-2026 and a Phase 3 study by early 2027. Phase 2b is where a drug is tested for efficacy in the target population at a selected dose to decide if it merits a large confirmatory trial; Phase 3 is the pivotal stage that compares the drug against the standard of care at scale and generates the bulk of data needed for an FDA approval filing. Positive Phase 3 results are typically the last major clinical step before a New Drug Application.
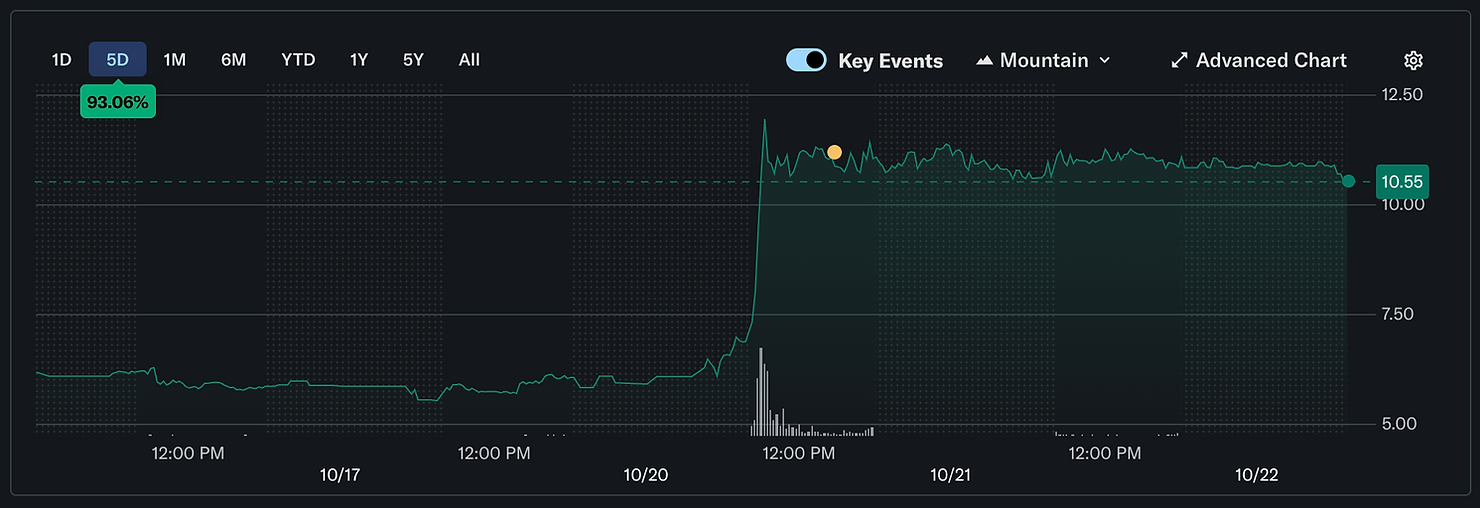
The stock market reacted sharply to the update, as shares of Alto moved roughly 80%, from $6 to near $11. This raise follows a run of prior financings. Alto closed a $45 million Series C round in November 2023, bringing the total equity raised to roughly $100 million. The company then listed on the NYSE in February 2024. The new $50 million placement extends the cash position and is explicitly tied to speeding ALTO-207 into late-stage studies.
Alto’s edge is how it treats brain data as a selection filter, not a dashboard. The platform collects EEG readings, performance on computerized cognitive tasks, and wearable-derived signals, then uses those measures to enroll likely responders and track changes in the domains that matter. This approach shifts trial math from population averages to stratified cohorts, which can raise observed effect sizes, cut noise, and shorten drug development timelines.
There are signs that Alto's biomarkers are maturing. In September, Alto reported an independent replication of an EEG biomarker that distinguishes patients with schizophrenia from healthy controls and correlates with cognitive performance.
Earlier in the year, the company also linked EEG signals to pharmacodynamic effects in depression and anhedonia, supporting the idea that these readouts are not just correlates but can track target engagement and predict responses. Together, that builds a spine for using brain signals as inclusion criteria and as secondary endpoints.
Precision psychiatry doesn’t necessarily eliminate the risk inherent in drug development; it changes its shape. Instead of betting on whether a drug works on average, the challenge becomes whether the biomarkers actually identify the right patients. The approach still has to show consistent results across clinics, time, and dosing.
Alto is proving that brain data is moving from an accessory to an invaluable infrastructure. The company is actively using EEG readings and cognitive measures to decide who gets into trials and how those trials are powered. Should Alto's approach improve effect sizes and reduce drug development timelines, it will turn EEG and digital phenotyping from a “nice to have” to a core aspect of any CNS drug development pipeline.
The business implications extend beyond Alto. As biomarkers become part of the pharma landscape, device and data firms may find clearer entry points as co-development partners rather than stand-alone ventures. Alto’s recent steps fit that direction: regulatory feedback on study design, Fast Track designation for its schizophrenia program, and financing linked to clinical execution.
In the near term, attention will center on whether the planned Phase 2b in treatment-resistant depression begins on schedule and whether biomarker logic is reflected in the final protocol. The outcomes of Alto's schizophrenia study, including analyses of replicated EEG signatures linked to cognition, will indicate whether their tools can scale beyond a single program.