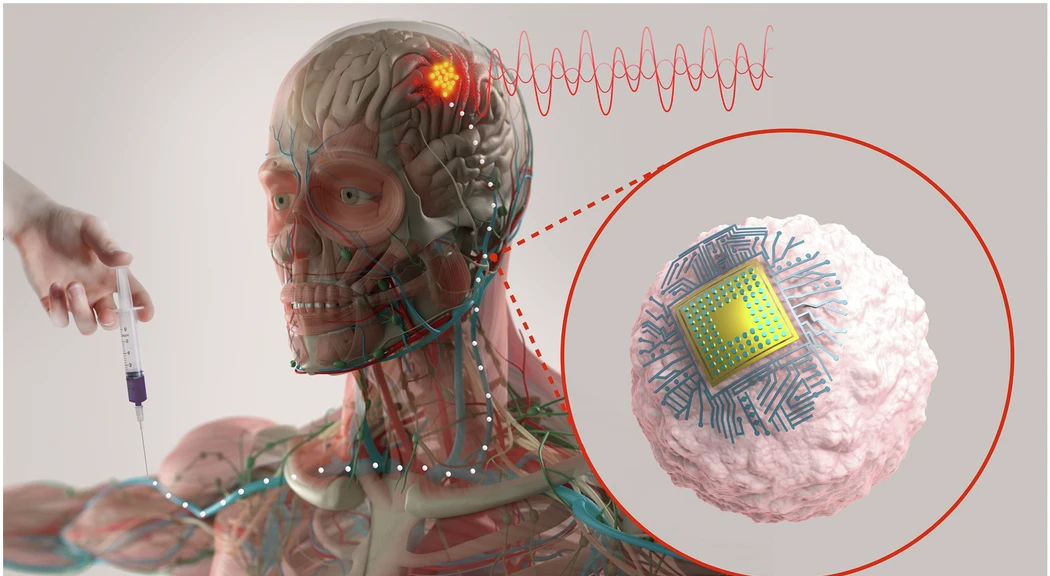
Today, a brain implant usually means an operating room, a neurosurgeon, and a hole in the skull. And that means recovery time and non-trivial surgical risk. In the work just published in Nature Biotechnology, an MIT-led team explores a different picture: microscopic chips hitch a ride on immune cells, are injected through an IV line, and then self-implant in an inflamed region deep in the brain. Once there, near-infrared light shone from outside the skull can wirelessly drive local neuromodulation.
The group calls the platform Circulatronics: cell-electronics hybrids that circulate through the bloodstream and settle in target tissue, offering a route to surgery-free neuromodulation. On the spectrum from EEG caps and consumer wearables at one end to open-skull BCIs and deep-brain stimulators at the other, Circulatronics aims for a new point on the curve, implants that behave like a biologic infusion but promise millimetre-scale precision. As the field moves towards non-invasive, MIT might have just introduced an invaluable approach.
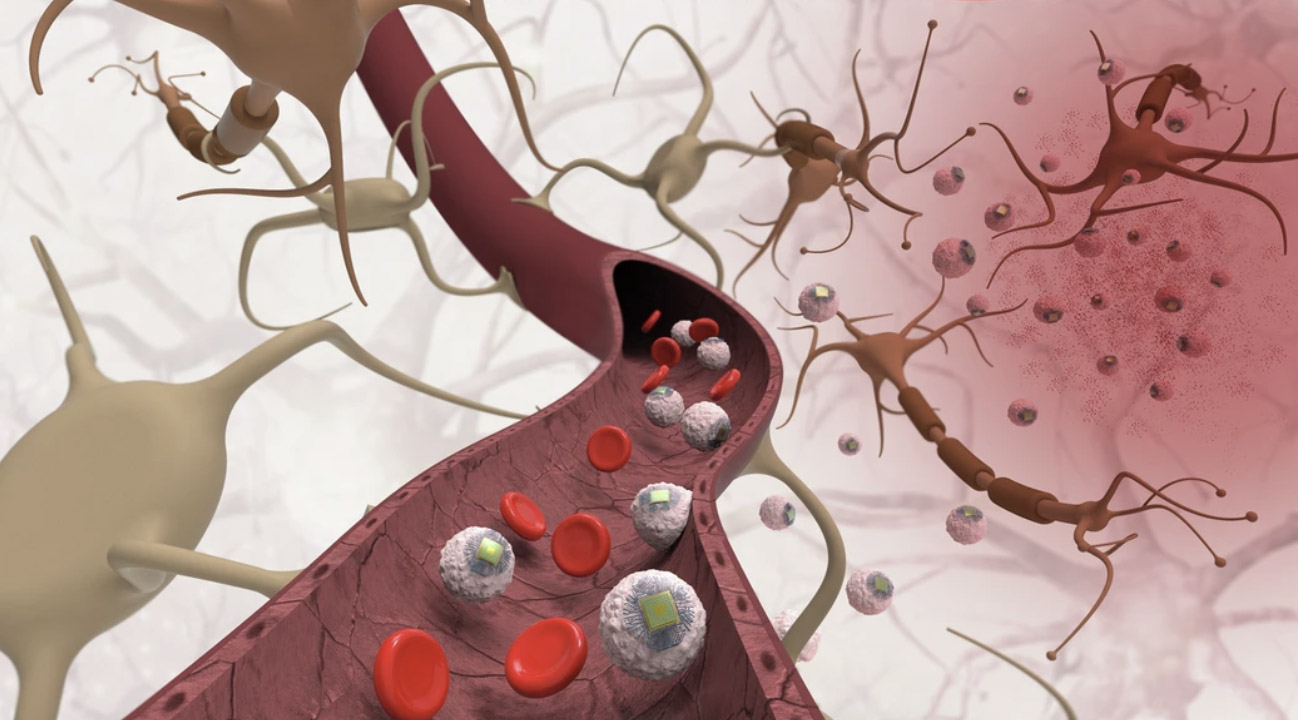
The MIT team’s BCI system begins with a tiny wireless implant called a SWED, a sub-cellular chip the size of a large bacterium. Each chip is built from organic semiconductor layers and titanium electrodes, small enough to drift through veins but powerful enough to generate a usable electrical pulse. Instead of placing the hardware through a burr hole or a catheter, the researchers attach the chips to monocytes, immune cells that naturally flow to inflamed tissue and can slip across the blood-brain barrier. Using a simple chemical bond, the team turns normal immune trafficking into a targeted delivery route.
To test their approach in vivo, the researchers created an inflammatory hotspot deep in a mouse thalamus and then infused the hybrid cells through a vein. Over the next three days, the monocytes migrated toward the injury, carrying the chips with them. When the animals were later examined, around fourteen thousand chips had accumulated around the inflamed region.
Once the chips were in place, the question became whether they could meaningfully affect neural activity. In a cell culture, a single SWED resting on a neuron was enough to trigger firing when illuminated, while the same near-infrared light on a bare cell did little. In mice, the team saw that same pattern at a larger scale: regions containing the chips lit up with activity when near-infrared light was applied. Electrode recordings added a functional layer, showing increases in firing in nearby neurons after each pulse. Controls did not show the same response, suggesting the effect came from the chips rather than from the illumination itself.
Alongside the core experiment, the study included preliminary safety testing. In vitro tests found no major impact on the viability of monocytes or neurons exposed to SWEDs. In live animals, blood panels and behavioural assays looked similar between treated and untreated mice, and tissue staining did not reveal device-specific injury. The cell carriers themselves cleared from the body within about ten days, while chips left in the brain in a separate experiment remained stable for months without obvious tissue damage. These are early signals rather than a clinical safety profile, but they help define the starting point for a technology that aims to enter the brain without surgery.
The MIT study includes several impressive milestones. A free-floating implant the size of a bacterium should not be able to generate enough current to influence a neuron, yet these sub-cellular chips do. And they do it without wiring or anchors, relying on non-invasive near-infrared penetration. The second leap is biological: instead of guiding hardware with a catheter, the system introduces immune cells as a cell-directed delivery route. It’s early, but those milestones form a coherent proof-of-concept, worthy of further development.
Placed on the broader BCI map, the work sits in a lane that barely existed until recently. Fully invasive systems offer precision and bandwidth but require neurosurgery and permanent implants. Non-invasive tools like EEG headsets or TMS avoid surgery but trade away spatial resolution and control. In between sits the new wave of minimally invasive BCIs, led by endovascular approaches such as Synchron’s Stentrode.
Circulatronics pushes that idea further, hinting at a new category, neither biological nor traditional device. And that category is already drawing commercial interest. A similar ‘cell plus electrode’ approach is actively being explored by Palo Alto start-up Subsense, a firm developing a nanoparticle BCI that is inserted nasally and stimulated through an external headset.
Where MIT’s approach could matter first is not in high-bandwidth BCI control but in modulating inflamed or dysfunctional tissue. The authors point to neurological conditions with clear inflammatory causes: stroke borders, MS lesions, brain tumours, neuropathic pain, and certain spinal or peripheral nerve injuries. In these settings, targeted modulation could influence repair processes. The concept also extends beyond the central nervous system. Immune cells home to affected joints, dorsal root ganglia, or painful lesions, areas already seeing interest in wireless peripheral nerve stimulators.
The translational hurdles remain substantial. Scaling from mouse skulls to human tissue makes light delivery far harder, and maintaining safe optical power in human subjects is a non-trivial challenge. Human inflammatory patterns are also more diffuse and variable. Safety questions arise quickly: millions of monocytes carrying chips raise microvascular and off-target concerns that rodent work cannot fully resolve. And critically, the current study shows neural activation, not symptom change.
Yet, the MIT result could reasonably be seen as a meaningful first step toward truly surgery-free neuromodulation from within the brain. The conceptual leap is genuinely new, and the approach is already accompanied by technical evidence showing that these particles can both target and modulate tissue, even if at small scales. Whether this stays a landmark paper or becomes a platform will depend on what survives the jump from mouse to human.
[Image credit: MIT - Yadav et al., 2025]